Support strong Canadian climate journalism for 2025
The United States will "work with the world" to distribute COVID-19 vaccines, President Donald Trump vowed Tuesday, even as he signed an order aimed at putting Americans at the head of the line.
In virtually the same breath, Trump delivered two diametrically opposed promises: to ensure U.S. citizens are first to reap the benefits of the country's effort to develop vaccines, but also to ensure other countries receive them as well.
"We're working very closely with other nations, to get the vaccines out to other nations; we're working with the world," Trump said.
With that, he signed an executive order "to ensure that American citizens have first priority to receive American vaccines," adding that the U.S. would begin working with other countries "almost immediately."
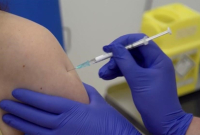
Ironically, it was the U.K. that became the first Western country Tuesday to administer a fully tested COVID-19 vaccine outside a clinical setting, doling out the Pfizer-BioNTech vaccine after British regulators approved it last week.
The U.S. Food and Drug Administration has offered a glowing assessment of the vaccine, saying it provides rapid, strong protection after the first of two doses and works well regardless of age, weight or ethnicity. U.S.-based Pfizer and Germany's BioNTech have already reported an efficacy rate of about 95 per cent after two shots.
There are also plenty more vaccines still in development, said Trump, promising they would "end the pandemic. The famously spotlight-hungry president might be keen to see the fruits of his development plan — dubbed "Operation Warp Speed" — deployed promptly beyond U.S. borders.
It wasn't abundantly clear how sharp-edged Trump's executive order would prove to be.
"We must ensure that Americans have priority access to COVID-19 vaccines developed in the United States or procured by the U.S. government," it reads.
"After ensuring the ability to meet the vaccination needs of the American people, it is in the interest of the United States to facilitate international access to U.S. government COVID-19 vaccines."
The order also requires national security officials to co-ordinate an interagency strategy for the implementation of international access to the vaccines within 30 days, provided a "sufficient supply" exists "for all Americans who choose to be vaccinated."
Dr. Raywat Deonandan, an epidemiologist and health sciences professor at the University of Ottawa, said limiting vaccine deployment around the world would have broader consequences beyond just the pace of immunization.
"No one is safe until everybody is safe," Deonandan said in an interview.
"Until the epidemic is stamped out in the darkest corners of Bangladesh, it is not over for everybody … so the argument can be made that it is not an advantageous position for anybody to be advocating for one population to get it before another."
Trump was under fresh political pressure following reports the White House passed up a chance to secure 500 million extra doses of Pfizer's vaccine. But that pressure didn't seem to spawn much fresh protectionist rhetoric.
North of the border, federal officials greeted the prospect of Trump's order with a collective yawn.
"We're very confident that Pfizer and other vaccine manufacturers that are contractually obligated to deliver vaccines to Canada will be able to meet those obligations," said Intergovernmental Affairs Minister Dominic LeBlanc.
"We have already assumed that we shouldn’t be tied to one particular manufacturing site, so the contracts contemplate that. Pfizer, for example, has many manufacturing facilities in Europe as well as the United States."
It wouldn't be the first time Trump has tried to deny medical resources to Canada as part of his "America First" doctrine.
In April, he tried to prevent U.S.-based 3M from honouring contacts with Canadian buyers of American-made N95 masks, vital in limiting the spread of the virus. The Minnesota company pushed back, eventually brokering a solution that involved using masks manufactured overseas to meet unprecedented U.S. demand.
Earlier this month, a Trump order authorizing states, pharmacies and wholesalers to import cheaper prescription drugs from Canada, part of his plan to tackle staggering drug costs in the U.S., went into effect.
But it was pre-emptively blocked by the federal Liberal government, which implemented a rule prohibiting the export of drugs meant for the domestic Canadian market that are either already in short supply or at risk of becoming scarce.
Dr. Jacalyn Duffin, a professor of medical history at Queen's University who writes a blog about Canada's chronic drug shortage problem, said the Trump administration has a clear track record of focusing on its own priorities.
"It just breaks my heart that there isn't solidarity out there in the United States … it's just being so selfish when it comes to the products that could help," Duffin said.
"We're all going to be going through the social distancing, the isolating and the mask-wearing for a long, long time, even when the vaccines make it to our shores."
This report by The Canadian Press was first published Dec. 8, 2020.




Comments